Background: Relapsed or refractory (R/R) acute leukemias (AL) represent major therapeutic challenges. Several reports have highlighted the deregulation of iron metabolism in various proliferative tumor models (and notably AL) which includes the over-expression of the transferrin receptor (TfR1/CD71). Our strategy is thus to harness CD71 overexpression to specifically target cytotoxic agents to leukemic cells. INA03 is an antibody drug conjugate (ADC) constituted of a humanized monoclonal IgG 4 against CD71 and MMAE. We herein report the preliminary safety and efficacy data of a phase 1 trial INA03 (NCT03957915) evaluating INA03 in R/R AL.
Methods: INA03 is given as 30 minutes IV infusion on days 1 (Loading dose,LD) followed by maintenance injections on D15 for cycle 1 and on D1 and D15 of cycle 2 and beyond. Eligibility criteria are age >18y, ECOG<2, R/R AL after at least one line of treatment with high dose chemotherapy and/or Vidaza and Venetoclax (VEN), LVEF>50%, Creatinine Clearance >30ml/min, normal liver function. The study design is based on a continuous reassessment method and includes 2 parts: a Loading Dose (LD) Titration study (Part 1, completed), followed by a Dose Escalation Part 2. During Part 1, sequential cohorts of 2 pts were included to receive ascending LD of INA03 followed by repeated fixed doses of INA03 to deal with a potential sink effect related to the high expression of CD71 in normal erythroblasts. During Part 2, sequential cohorts of 3 pts received escalating doses of INA03 Q2 weeks in 28-day cycles. The objectives were to establish the maximal tolerated dose (MTD) for subsequent administration and both safety/tolerability and anti-leukemic activity.
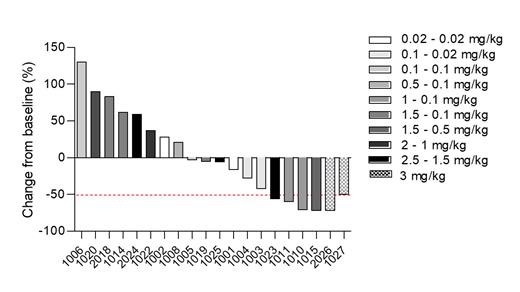
Results: With a cutoff date of July 05, 2023, 27 patients (pts) across 10 cohorts were included in the study. The median age was 73 (range: 39 - 83), 25 (93%) pts had AML and two (7%) had ALL. Four (14.8%) pts had a prior allogeneic stem cell transplantation and 18 (66.6%) had prior VEN exposure. Pts received escalating doses of INA03 of 0.02 mg/kg to 3 mg/kg. Two patients in the 3 mg/kg loading dose cohort presented a dose limiting toxicity (DLT), one with grade 3 toxic erythema, grade 3 hepatitis and grade 3 mucositis and one with grade 4 colonic perforation. Up to the 2.5 mg/kg dose, no grade 2-4 treatment emerging adverse events (TEAE) were observed among the 19 evaluable pts and only one related grade 1 TEAE (hyperkalemia/phosphatemia) was reported. Transient decrease of reticulocyte count and erythroblasts percentage were observed at 0.5 mg/kg and above. Blasts reductions were seen in 6/20 (30%) evaluable pts at dose > 1 mg/kg including two partial responses according to ELN 2022 classification. MMAE pharmacokinetics (PK) appears dose-proportional with a small accumulation after repeated IV, while INA03 PK exhibits target-mediated drug disposition without accumulation.
Conclusions: The preliminary results of this phase 1 study indicate that INA03 is well tolerated for doses up to 2.5 mg/kg. Induction of transient erythroblastopenia indicates effective targeting of CD71 in vivo and early signs of antileukemic efficacy have been observed in patients with refractory AL. Inclusion in the trial are continuing and additional data will be presented.
Disclosures
Bories:Kite Gilead: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Récher:Iqvia: Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria; Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Boehringer: Membership on an entity's Board of Directors or advisory committees. Belanger:Inatherys: Current Employment. Monteiro:Inatherys: Current Employment. Hermine:AB Science, BMS/Celgene, Alexion, Novartis, and Inatherys: Research Funding; AB Science: Consultancy, Other: Shareholder. Launay:Inatherys: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal